Enhancing Patient Care with the Design, Validation and Compliance of Smart Edge Medical Devices
AI-Driven Medical Device Engineering Services for Smarter Healthcare
The fast-evolving medical devices and healthcare industry is facing several challenges in designing and productizing new concepts like connected devices, remote patient monitoring, edge processing, integration with EHR systems, and evolving FDA and regulatory compliance standards — all while continuing to support patient care using legacy devices and outdated infrastructure. With increasing reliance on AI, IoT, and cloud-based solutions, MedTech Companies must ensure precise, efficient, and secure patient care.
ACL Digital can be the right technology partner in such circumstances for medtech companies — from enterprise-grade companies to growth-stage startups. ACL Digital brings extensive experience in delivering next-gen products to healthcare automation industry from concept to manufacturing readiness, with the necessary expertise and consulting for medical devices engineering in areas such as HIPAA compliance, GDPR, ISO 13485, IEC 62304, and IEC 60601-1/2/6 across the product development life cycle for FDA Class I, II, and III devices.
Our experience spans various device categories, including diagnostics and IVD, therapeutic, surgical, local and remote monitoring, vision, personal protection, home healthcare, neurostimulation, wearables, and hospital assistance.
Revolutionize Care with Our Medical Devices Offerings
Medical Product Engineering
We design and develop medical devices, ingestible sensors, and wearables to monitor patient health and enhance diagnostics.
Technology Integration
We integrate AI, Edge ML and secure boot technologies with medical devices to boost efficiency and healthcare innovation.
Value Engineering
We optimize costs through Value Engineering, delivering smarter alternatives that boost margins, speed adoption, and reduce time-to-market.
Connectivity Support
We enable medical devices with BLE, Wi-Fi, UWB and 5G connectivity. Ensuring seamless communication and real-time secure data sharing is our priority.
Tools Expertise
We use tools like VectorCast for requirement traceability. We achieve over 95% code coverage for superior software reliability.
Quality & Compliance
We provide end-to-end testing services, QMS setup, covering test strategy, test plan, requirement traceability, dry runs, formal execution, clinical trials, FDA 510K.
Addressing Key Challenges in the MedTech Industry
Remote Medical Assistance
Develop IoT healthcare automation services to remotely monitor patient health and data history on iOS and Android devices.
Technology Integration
Transform legacy health monitoring devices through advanced technology integration into connected, intelligent systems for faster, more accurate, and data-driven diagnostics.
Risk Management and Cybersecurity
Embed end-to-end cybersecurity and risk management frameworks to safeguard patient data, ensure device integrity, and meet regulatory standards.
Medical Regulation Compliance
Ensure FDA and HIPAA compliance to meet regulatory standards and reduce risks in healthcare solutions.
Why Choose ACL Digital to Accelerate Medical Device Innovation

One-Stop Service Provider
As a healthcare solution provider, we excel at all stages of medical-grade device development, application development, testing, large-scale manufacturing, and cloud integration.
Regulatory Experience
We conduct We conduct pre-compliance design and testing (HIPAA, GDPR, FDA Class I, II, and III,IEC 62304, and IEC 60601-1/2/6, ISO 14971, HITRUST NIST, HITRUST CSF, FHIR, EU-U.S. & Swiss-U.S. Privacy Shield, and IEC).
Proven Domain Expertise
We bring deep knowledge and proven expertise in transforming legacy medical devices such as radiology apparatus, monitoring/diagnostic, respiratory, and endoscopy to smart ones. We have engineered over 10+ FDA-compliant products aligned with key regulatory standards.
Collaboration
We maintain solid partnerships with hardware and pre-complaint medical standard cloud platform providers to facilitate faster time to market.

Why Choose Medical Automation
One-stop Service Provider

Regulatory Experience

Proven Domain Expertise
Collaboration

Client Impact

Revolutionizing Endoscopy with a HIPAA-Compliant Cloud Solution
A healthcare provider implemented a secure, scalable, and HIPAA-compliant AWS cloud-based solution to enhance data privacy and enable seamless remote monitoring for endoscopic procedures.
The Challenges
- Lack of Cloud Infrastructure: The absence of a cloud-based environment hindered scalability and flexibility.
- Inadequate Security Measures: Ensuring secure access to sensitive medical information was critical but insufficient in the existing setup.
The Outcomes
- Delivered a HIPAA-compliant, user-ready solution that facilitated faster market entry.
- Enhanced scalability, security, and access control for sensitive healthcare data.
- Enabled remote patient monitoring for improved operational efficiency.
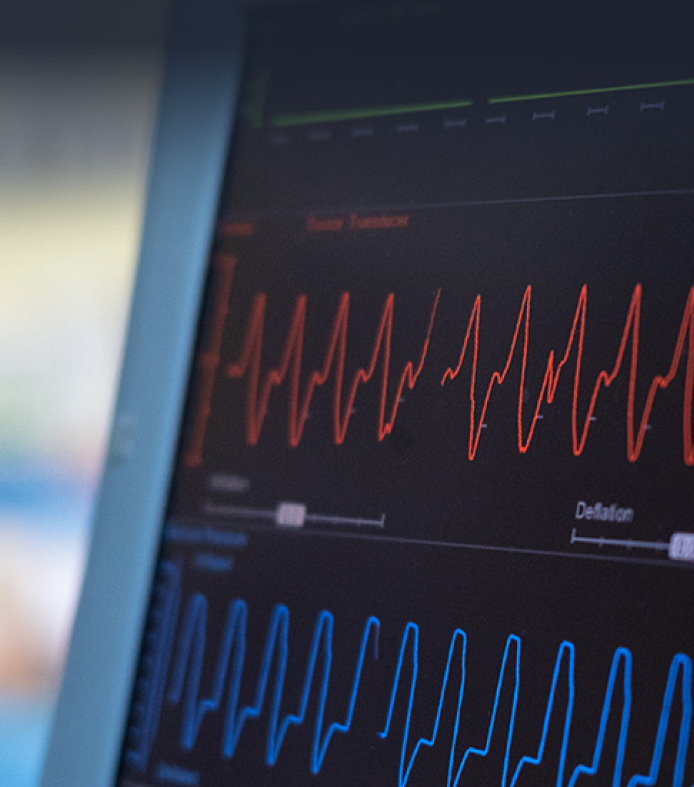
Ensuring FDA Approval with Robust Firmware Testing
ACL Digital provided expert firmware testing services using VectorCAST for a medical device manufacturer to meet FDA approval requirements.
The Challenges
- DA approval for a class-C medical device
- Testing device firmware using VectorCAST
- Generating reports for FDA submission
The Outcomes
- Reduced compliance failure risk with VectorCAST testing
- Improved code accuracy to 90%
- Achieved FDA approval for the medical device

Enhanced Health Monitoring Solution with ACL Digital’s Solutions
We provided a customizable CENTAURI 200 gateway to integrate with a home monitoring solution for a US-based healthcare company.
The Challenges
- Integrating a home monitoring solution with a cloud-based platform
- Migrating existing applications and functionalities quickly
The Outcomes
- Accelerated solution launch by 50% with CENTAURI 200
- Expanded market reach with LTE functionality



