

ACL Digital
The Curious Case of Re-Screened Subjects
In clinical trials, re-screening is allowed wherein subjects are permitted for rescreening if an individual has failed in an earlier attempt. Meanwhile, some subjects fail the screening process due to not meeting inclusion/exclusion criteria. Such individuals who fail screenings will be screened again upon completion of a specific time or meeting a specific criterion as per the protocols and regulatory guidelines. Re-screening is beneficial for both the individual and the sponsor. In case of rare diseases, it becomes beneficial where patient population is limited and hard to recruit. Also, when the clinical trial opts for re-screening, some intriguing questions arise, making the re-screening procedure a curious case.
- What should be the subject identifier (USUBJID) for the re-screened subject?
- How do we trace a subject who has passed screening after multiple attempts?
- What will be the implications on DM?
- What are the standards (SDTMIG) for re-screening?
Let us have a look into these questions and get a deeper insight.
What should be the subject identifier (USUBJID) for the re-screened subject?
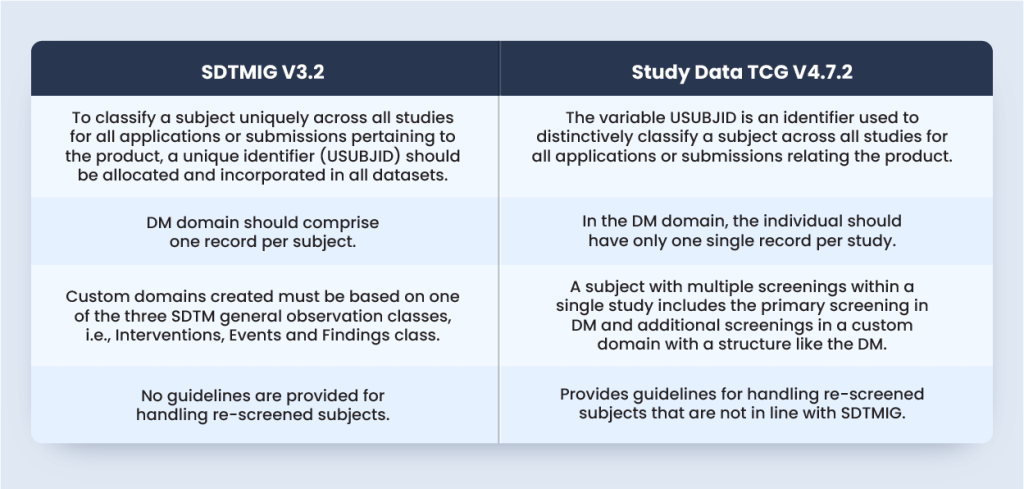
According to SDTMIG V3.2 Section 4.1.2.3, to ascertain a subject distinctly across all studies for all applications or submissions affecting the product, a unique identifier (USUBJID) should be allocated and incorporated in all datasets.
As per Study Data Technical Conformance Guide (TCG) V4.7.2 Section 4.1.1.2, the variable USUBJID is an identifier used to distinctively identify a subject across all studies for all applications or submissions involving the product.
From the guidelines, USUBJID should be unique for each subject irrespective of whether the individual is re-screened or not.
How do we trace a subject who has passed screening after multiple attempts?
Although SDTMIG does not provide guidelines for handling re-screened subjects, TCG Section 4.1.1.2 mentions that the variable Subject Identifier (SUBJID) distinctively identifies the individual that takes part in a study. If an individual is screened or enrolled more than once, then the subject’s SUBJID should be unique for every screening or enrolment.
From the guidelines, one can infer that there should be a one-to-one relationship between USUBJID and SUBJID for subjects that appear only once in a study for screening. However, a one-to-one relationship is not applicable for re-screening studies, and the SUBJID can be different for each screening of the same individual. Thus, SUBJID is the variable used to trace re-screened subjects.
What will be the implications on DM?
As per SDTMIG V3.2 DM domain should comprise one record per subject. The same is reiterated in the TCG Section 4.1.1.3 as, in the DM domain, each subject should have only a single record per study.
Suppose if we consider a hypothetical scenario:
- Example study is ABC123. A Phase II Multicentre, Double-Blinded, Placebo-Controlled to evaluate efficacy and safety of subjects with moderate symptoms of XYZ disease
- Subjects are permitted to re-screen multiple times
- Subject 020 from site #1 fails screening and SUBJID = “01-005”
- The same subject returns to screening again at same site #1 with different SUBJID = ”01-020” and gets screened in second attempt by meeting all IE criteria
- As per the guidelines, during both screenings, the subject will have the same unique subject identifier (USUBJID) that is of primary screening, i.e., attempt in which the subject got successfully screened in:
USUBJID = “ABC123-01-020”
According to this scenario, the DM domain will appear as below for the subject considered:
dm.xpt
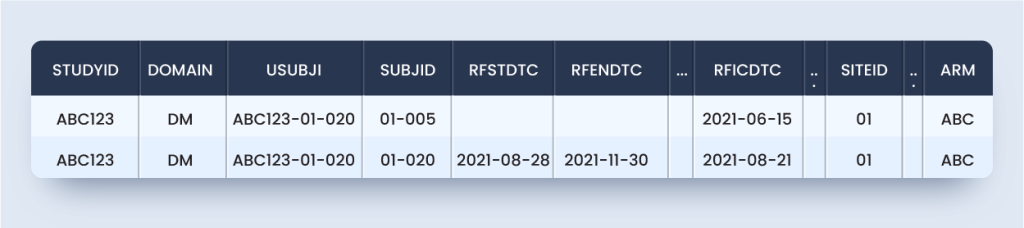
But the above domain is not SDTMIG or TCG compliant because it is not one record per subject.
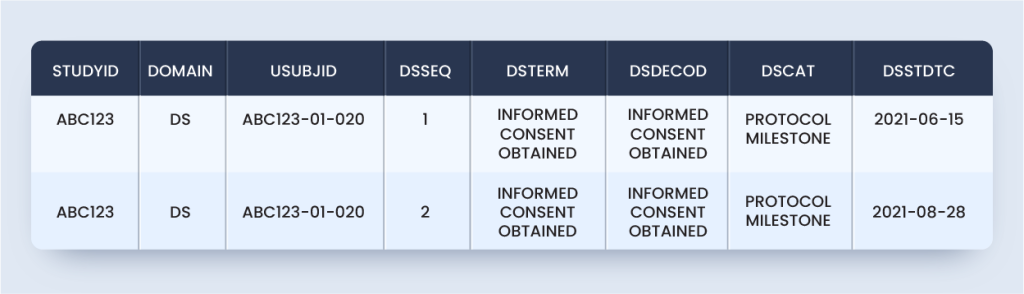
However, according to the TCG Section 4.1.1.3, the subject with numerous screenings within a single study includes the preliminary screening in DM with further screenings in a custom domain with a structure like the DM.
Now, as per this guideline DM domain and ZM custom domain will appear as below for the subject considered (Approach #1):
dm.xpt
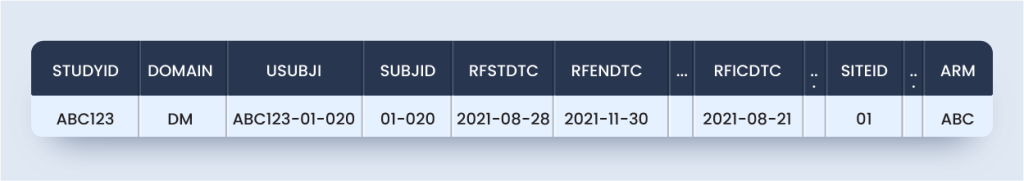
zm.xpt
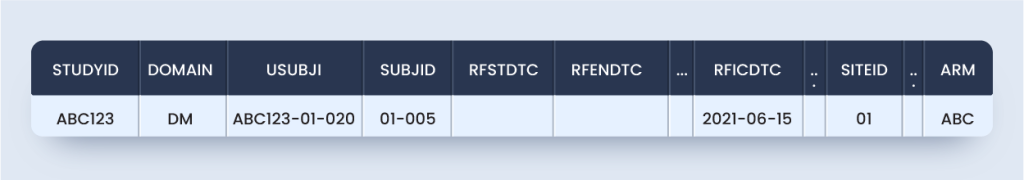
Approach #1 is the most TCG compliant but not SDTM compliant because SDTMIG V3.2 Section 2.6 Custom domains must rely on one of the three SDTM general observation classes, i.e., interventions, events, and the findings class.
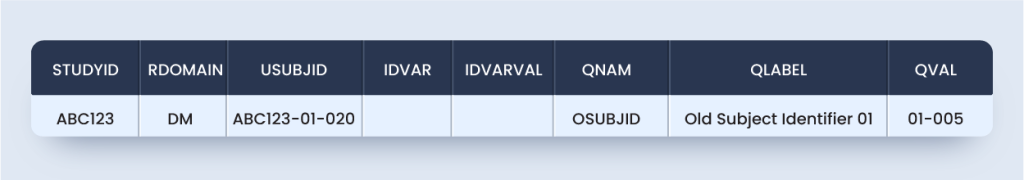
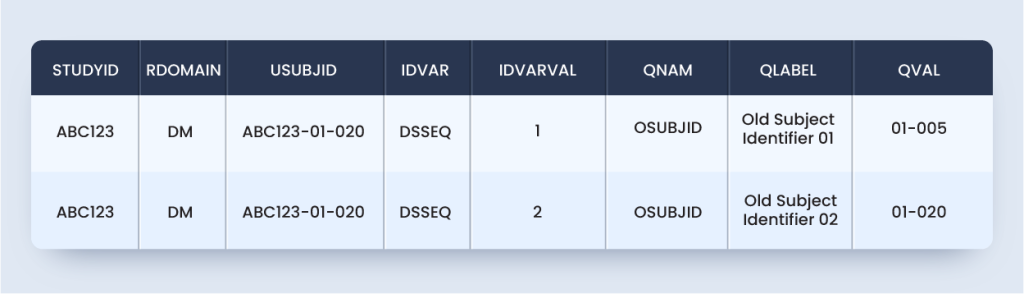
So, an alternate approach (Approach #2) that ensures SDTMIG and TCG compliant includes the primary screening in DM with additional screenings in SUPPDM is as below:
dm.xpt
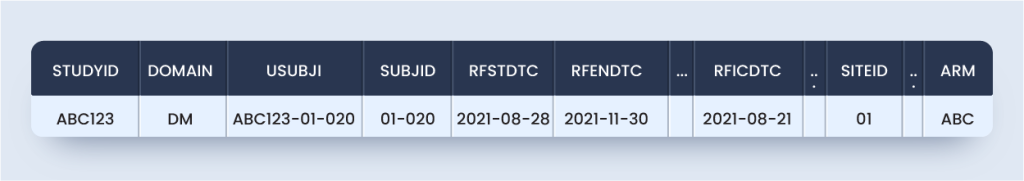
suppdm.xpt
ds.xpt
suppdm.xpt
What are the standards (SDTMIG) for re-screening?
SDTMIG V3.2 does not provide any guideline for handling re-screened subjects. However, the Study Data TCG sheds light on the orientation towards the submission and handling of the re-screened individuals. The below table will summarize the standards from both SDTMIG V3.2 and Study Data TCG V4.7.2:
Conclusion
From the sponsor’s perspective, allowing re-screening of the subjects in clinical trials has increased due to its cost-efficiency. Also, the nature of the disease (rare disease) or complex inclusion/exclusion criteria may increase the difficulty to find appropriate individuals for the study. Re-screening makes sense to an individual who was not screened earlier because of minor conditions, and with elapse of time, the subject is sure to meet IE criteria. Therefore, re-screening is beneficial for both the individual and the sponsor.
SDTMIG does not provide any formal guidelines for the submission of re-screened subjects. Although technical regulations do provide recommendations, they are not CDISC compliant. So, we can conclude that both approaches, #1 or #2 (as per TCG or PhUSE WG), are acceptable. However, one should acknowledge both methods in the SDTMRG.
Approach #1 takes care of DM with the Custom domain, but other SDTM parts try to capture data related to re-screening of the same individual. Here, one can use the xxREFID variable with a value equal to SUBJID from each screening.
Approach #2 can use Supplemental Qualifiers for other domains, like (SUPPDS) to capture the data related to re-screening of the same subject.
On a general note, we recommend you adopt an approach that is SDTM-compliant until the availability of formal guidelines. It accelerates the drug approval process as reviewer tools used by regulatory authorities are developed based on the SDTMIG. The consultants at ACL Digital take a pro-active business-centric approach in streamlining the data environment, with a progressive and agile approach. Contact us today for more information.
Source Attribution
Related Insights




Top 5 Hard Truths About Your Governance Strategy

ETL Simplified: Storing and Transforming Data Fully Inside Databricks

