Dosage Infusion Pump Firmware Development & Quality Engineering for a Healthcare Company in the US

Overview
The client is a US based leading pharmaceutical company. They wanted to develop a FDA compliance medical Infusion pump device to automate and control desired medical dosage. ACL developed infusion pump embedded software application with integrated sensors to monitor and control the flow of water/medicine fluid dosage.
Download Case Study
Challenges
Develop a FDA compliance medical Infusion pump device
Automate and control desired medical dosage
Reduce human errors in dosage infusion
Solution
- Developed infusion pump embedded software application
- Integrated sensors to monitor and control the flow of water/medicine fluid dosage
- Dosage configuration for desired therapy
- Developed GUI for 100+ screens using MICRIUM
- Monitor battery consumption & infusion pump with audible alarms and notifications
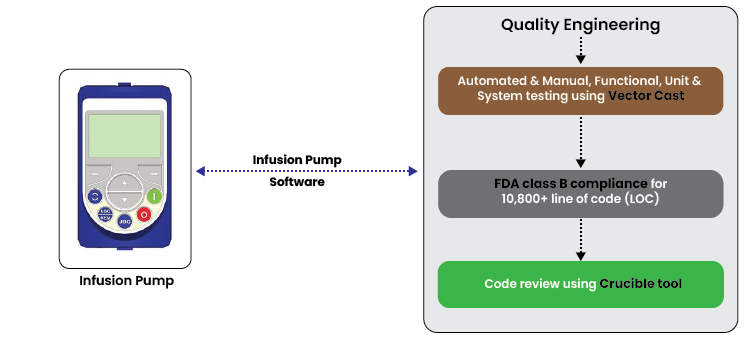
- Automated & Manual, Functional, Unit & System testing using Vector Cast
- FDA class B compliance for 10,800+ line of code (LOC)
- Code review using Crucible tool
- ~1000 test cases to verify use cases-based functionality
- Detect violation and fix them as per the code compliance guideline & PC Lint tool
Outcomes
- Enhanced operational accuracy and reduced errors with quality engineering and testing
- Enabled FDA Compliance for 10K+ Line of Code
- Intuitive GUI development for ease of use of product